81. Congenital adrenal hyperplasia Disease details / Clinical trials / Drug dev / DR info
Clinical trials : 102 / Drugs : 72 - (DrugBank : 18) / Drug target genes : 14 - Drug target pathways : 71
| Other names | CAH;Congenital adrenal enzyme deficiency;Congenial adrenal cortex enzyme deficiency; | ||
|---|---|---|---|
| Disease group | Endocrine diseases | ||
|
Domestic patients
Med expenses recipients (FY2024) |
1,113 patients Age distribution
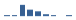 💬 💬 |
||
| Related info (in Japanese) | - | ||
| Clinical research form [April 1, 2025 ~] (in Japanese) MHLW Intractable Diseases (in Japanese) | Overview, diagnostic criteria, etc. (pdf), Clinical research form (pdf) 081-01, 081-02, 081-03, 081-04, 081-05, 081-06 | ||
| Specific pediatric chronic diseases, Japan (in Japanese) | 5-25-56. Go to site. | ||
| Subtypes | No. | Name | Specific pediatric chronic diseases, Japan (in Japanese) |
| 81-1. | Congenital Lipoid Adrenal Hyperplasia; | 5-25-50. Congenital lipoid adrenal hyperplasia | |
| 81-2. | 3β-Hydroxysteroid Dehydrogenase Deficiency; | 5-25-51. 3 beta-hydroxysteroid dehydrogenase deficiency | |
| 81-3. | 21-Hydroxylase deficiency;21-OHD; | 5-25-54. 21-hydroxylase deficiency | |
| 81-4. | 11β-Hydroxylase deficiency; | 5-25-52. 11 beta-hydroxylase deficiency | |
| 81-5. | 17α-Hydroxylase deficiency; | 5-25-53. 17 alpha-hydroxylase deficiency | |
| 81-6. | P450 oxidoreductase deficiency; | 5-25-55. P450 oxidoreductase deficiency | |
